Abstract
Introduction: Venous thromboembolism (VTE) is a common cause of morbidity and mortality among patients with multiple myeloma (MM). Thromboprophylaxis is a safe and effective way to decrease VTE in other high-risk populations. Current guidelines recommend use of thromboprophylaxis in MM patients at high-risk of VTE, but no validated model predicts VTE in MM. A risk prediction model for VTE in MM would allow for use of thromboprophylaxis in MM patients at high-risk of VTE while sparing those at low risk. Therefore, we sought to develop a risk prediction model for VTE in MM.
Patients and Methods: Using a nationwide cohort of Veterans, we identified 4,448 patients diagnosed with MM between 1999 and 2014. We retrospectively followed patients for 180 days after start of MM chemotherapy. We identified candidate risk factors through literature review for inclusion into the time-to-event models. We used the methods of Fine and Gray to model time to VTE while accounting for the competing risk of (non-VTE) death. To minimize immortal time bias, all treatment variables were entered as time-varying variables. Using a backward, step-wise approach, we retained variables in the model with a p ≤ 0.05, or with a p < 0.50 with findings consistent with prior literature. Using beta coefficients, we developed a risk score by multiplying by a common factor and rounding to the nearest integer. The risk score for each patient was the sum of all scores for each predictor variable. We assessed model performance with Harrell's c-statistic and with the inverse probability of censoring weighting approach. Through bootstrap analysis, we validated the model internally. We carried out all statistical analyses using SAS version 9.4 (SAS Institute, Cary, NC).
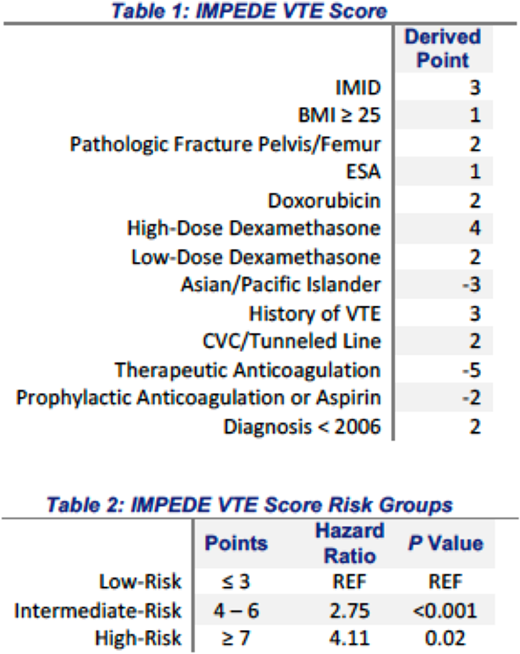
Results: The median time from MM diagnosis to start of treatment was 37 days. A total of 53 patients (5.7%) developed VTE within 6 months after start of MM-specific therapy. The mean time from chemotherapy start to VTE was 69 days, with 69% of VTE events occurring in the first 3 months of chemotherapy. The factors associated with VTE were combined to develop the IMPEDE VTE score (IMID 3 points, BMI 1 point, Pathologic fracture pelvis/femur 2 points, ESA 1 point, Dexamethasone (High-dose 4 points, Low-Dose 2 points)/Doxorubicin 2 points, Ethnicity/Race= Asian -3 points, history of VTE 3 points, Tunneled line/CVC 2 points) (Table 1). In addition, use of therapeutic anticoagulation (-5 points) with warfarin or low molecular weight heparin (LWMH) and use of prophylactic LMWH or aspirin (-2 points) were associated with a decreased risk of VTE. The model showed satisfactory discrimination in both the derivation cohort (Harrell's c-statistic = 0.66) and in the bootstrap validation, c-statistic = 0.65 (95% CI: 0.62 - 0.69). Using three risk groups, the incident rate of VTE with the first 6-months of starting chemotherapy was 3.1% for scores ≤ 3 (low-risk), 7.5% for a score of 4-6 (intermediate-risk), and 13.3% for patients with a score of ≥ 7 (high-risk). The risk of developing VTE within 6 months after starting chemotherapy was significantly higher for patients with intermediate- and high-risk scores compared to low-risk (Table 2).
Conclusions and Relevance: We developed a risk prediction rule, IMPEDE VTE, which can identify patients with MM at high-risk of developing VTE after starting chemotherapy. IMPEDE VTE could guide clinicians in selecting patients for thromboprophylaxis in MM.
Sanfilippo:BMS/Pfizer: Speakers Bureau. Wang:Daiichi Sankyo: Consultancy, Other: Travel. Wildes:Janssen: Research Funding. Mikhael:Onyx, Celgene, Sanofi, AbbVie: Research Funding. Carson:Flatiron Health: Employment; Washington University in St. Louis: Employment; Roche: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal